Search
Research
BANK CF: The Respiratory Centre BIOBANKThe Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) program has grown from an early surveillance program initiated in Perth in 1999, which performed bronchoalveolar lavage (BAL) to evaluate pulmonary infection and inflammation, as well as infant lung function testing.
Research
BPG Formulation Preferences Study: Exploring patient, family and clinician reformulations preferences for BPGThe key objective of this study is to collect data about patient and clinician preferences about reformulations.
Research
Building social and emotional wellbeing through the artsThe ‘Building Social and Emotional Wellbeing Through the Arts Project’ was funded in 2021 by Healthway and supported through a partnership between The Kids Research Institute Australia and Edith Cowan University (ECU).
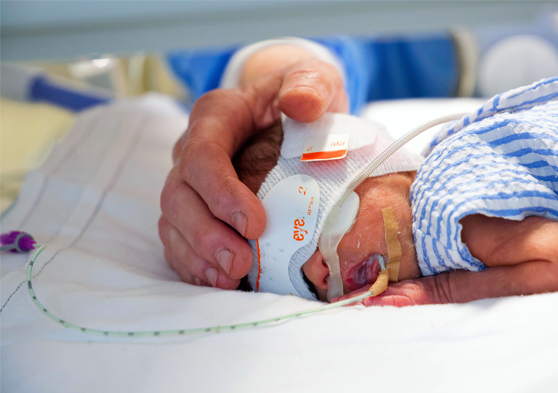
The CIRCA DIEM study aims to establish if cycling environmental light and noise levels for premature infants during their initial hospital stay leads to earlier development of circadian (daily) rhythms and better outcomes for the preterm babies, including improved brain development.
Research
Co-designing a trauma-informed program for parents whose infant has had a Neonatal Intensive Care Unit (NICU) admissionHaving a newborn child admitted into a NICU can be highly traumatic for parents. The compounding effects of the NICU clinical environment, having a seriously ill child, in addition to the inability to care or adequately bond with your child can be extremely distressing.
Research
Defective cell migration as a mechanism of dysregulated asthmatic airway repairThe findings from this study show that in children with asthma this protective barrier is different from children without asthma.
Research
Determinants and Outcomes of Preterm Birth & Pathways into Developmental DisordersBrad Carrington Fiona Farrant Shepherd Stanley BSc (Hons), PhD PhD FAA FASSA MSc MD FFPHM FAFPHM FRACP FRANZCOG HonDSc HonDUniv HonFRACGP HonMD
Research
Development of a longer acting formulation of Penicillin G for the treatment and prevention of acute rheumatic fever and rheumatic heart diseaseThis project aims to develop a longer acting formulation of penicillin, such that frequency of the injection can be increased up to 3-6 months.
Research
Djaalinj Waakinj Ear Portal: An ENT and Audiology referral pathway for improving access to ear and hearing services for Aboriginal children in the metropolitan area using telehealthThe Djaalinj Waakinj (Listening, Talking) Ear Portal project commenced in 2020 to evaluate an equitable ear and hearing care pathway for Aboriginal children residing in the metropolitan area of Perth.
Research
Djaalinj Waakinj: A cohort study of otitis media in young urban Aboriginal children – prevalence, risk factors and consequencesChris Deborah Peter Natasha Valerie Brennan-Jones Lehmann Richmond Morrison Swift PhD AO, MBBS, MSc MBBS MRCP(UK) FRACP Head, Ear and Hearing Health
