Search
Research
COVID-19 and Behavioral SciencesCitation: Jackson T, Steed L, Pedruzzi R, Beyene K, Chan AHY. Editorial: COVID-19 and Behavioral Sciences. Front Public Health. 2022;9. Keywords:
Research
Consensus guidelines for the diagnosis and management of invasive aspergillosis, 2021Invasive aspergillosis (IA) in haematology/oncology patients presents as primary infection or breakthrough infection, which can become refractory to antifungal treatment and has a high associated mortality. Other emerging patient risk groups include patients in the intensive care setting with severe respiratory viral infections, including COVID-19.
Research
Weighing the Risks of Perimyocarditis With the Benefits of SARS-CoV-2 mRNA Vaccination in AdolescentsChristopher Blyth MBBS (Hons) DCH FRACP FRCPA PhD Centre Head, Wesfarmers Centre of Vaccines and Infectious Diseases; Co-Head, Infectious Diseases
Research
Evaluation of protocol amendments to the Environmental Determinants of Islet Autoimmunity (ENDIA) study during the COVID-19 pandemicLiz Davis MBBS FRACP PhD Co-director of Children’s Diabetes Centre Co-director of Children’s Diabetes Centre Professor Davis is a paediatric
Research
Interrupted time-series analysis showed unintended consequences of non-pharmaceutical interventions on paediatric hospital admissionsCOVID-19-associated non-pharmaceutical interventions (NPI) have disrupted respiratory viral transmission. We quantified the changes in paediatric hospital admissions in 2020 from five different NPI phases in Western Australia for acute lower respiratory infections (ALRI) in children in the context of all-cause admissions.
Research
COVID-19 vaccine Mandates: An Australian attitudinal studyThe rollout of vaccines against COVID-19 is prompting governments and the private sector to adopt mandates. However, there has been little conceptual analysis of the types of mandates available, nor empirical analysis of how the public thinks about different mandates and why. Our conceptual study examines available instruments, how they have been implemented pre-COVID, and their use for COVID-19 globally.

News & Events
Sophisticated modelling shows WA’s Omicron outbreak still has months to runWestern Australia’s Omicron outbreak is far from over, with new modelling showing the number of total infections is only at its half-way point.
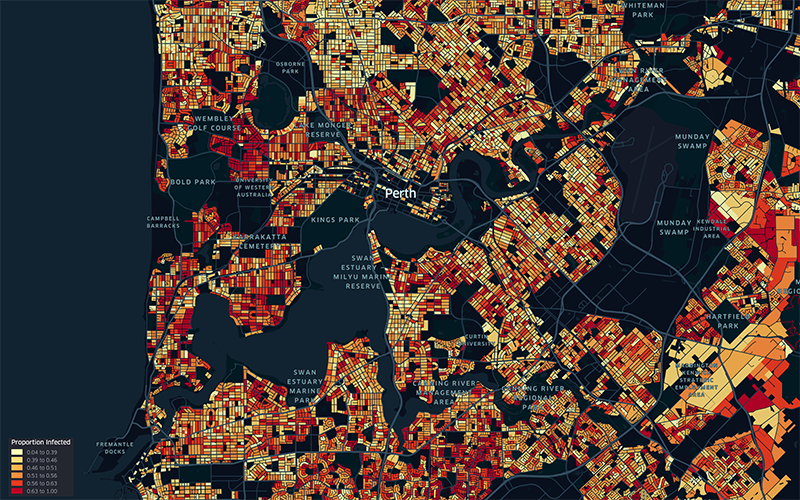
News & Events
Sophisticated new modelling suggests keeping mask mandate could prevent 147,000 COVID-19 casesWA’s current Omicron COVID-19 outbreak could jump by 147,000 cases if mask mandates are abandoned before the Easter long weekend, according to sophisticated new modelling.
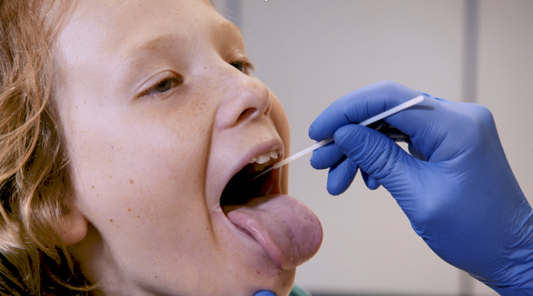
News & Events
COVID tests well tolerated by WA school kidsA study conducted across 40 WA schools has found COVID-19 testing using a combined nose and throat swab was well tolerated by children as young as 4 years.

News & Events
Assessing COVID-19 Across Western Australian SchoolsWestern Australia has been highly successful at containing community spread of COVID-19 to date.
