Search
Research
RSV prophylaxis use in high-risk infants in Western Australia, 2002-2013: a record linkage cohort studyThe monoclonal antibody, palivizumab is licensed for use in high-risk infants to prevent severe illness caused by respiratory syncytial virus (RSV). The level of its use and compliance with current jurisdictional guidelines which were amended in 2010, is unknown.
Research
Lactoferrin Expression Is Not Associated with Late-Onset Sepsis in Very Preterm InfantsPreterm infants are at a high risk of developing late-onset sepsis (LOS). Lactoferrin is one of the most abundant endogenous antimicrobial proteins expressed in breast milk, stools, and blood, and a candidate for preventive intervention. Large clinical trials have recently investigated whether enteral supplementation with bovine lactoferrin reduces LOS.
Research
Clinical protocol for a longitudinal cohort study to identify markers of vaccine immunogenicity in newborn infants in the gambia and papua New GuineaImmunity is distinct in early life and greater precision is required in our understanding of mechanisms of early life protection to inform development of new pediatric vaccines
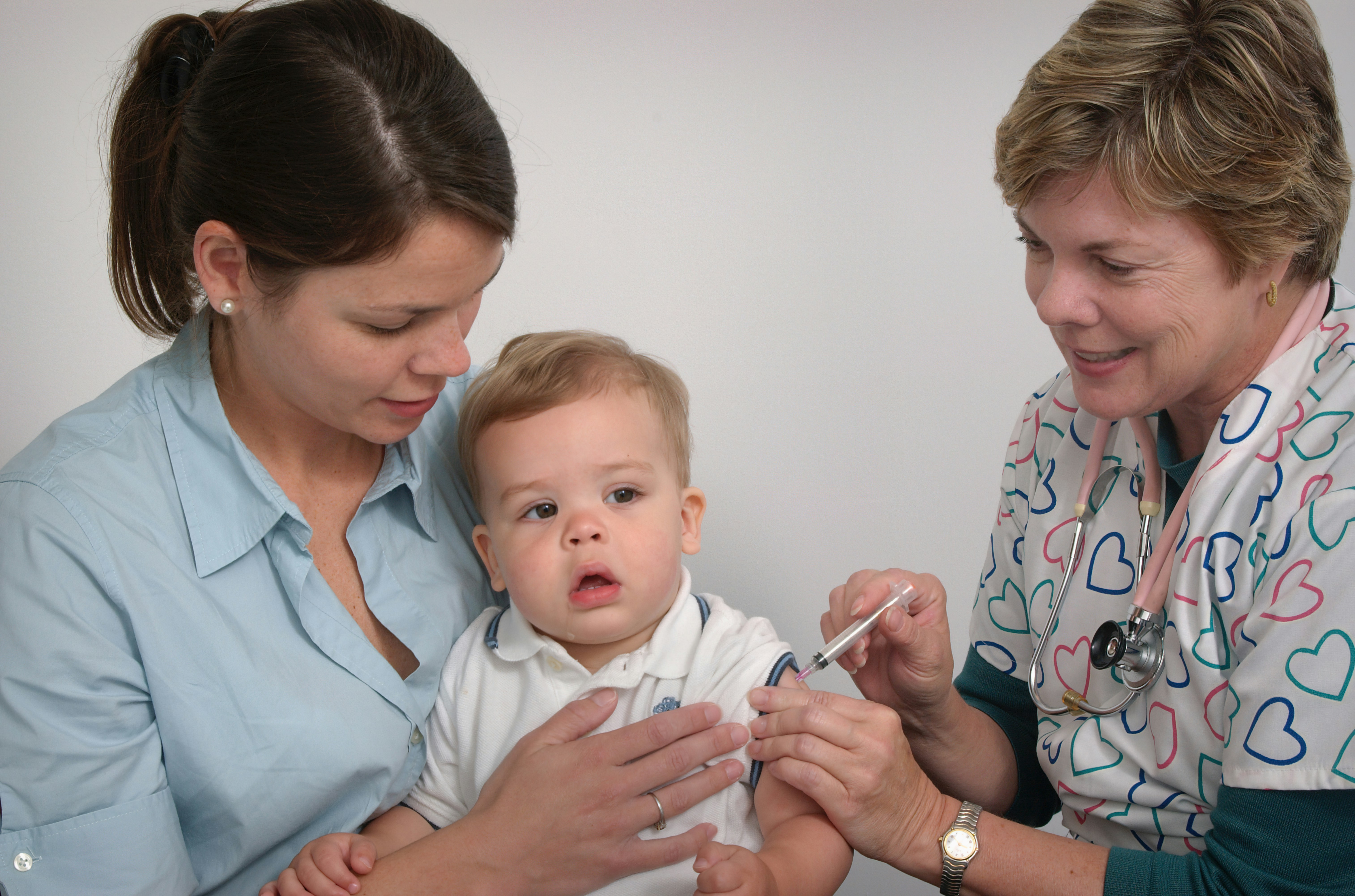
News & Events
The Kids researchers help quantify global impact of life-saving vaccinesResearchers at The Kids Research Institute Australia have helped map the global impact of life saving vaccines to mark the 50-year anniversary of the Expanded Programme on Immunisation (EPI).
Research
The Use of Test-negative Controls to Monitor Vaccine Effectiveness: A Systematic Review of MethodologyOur review highlights similarities and differences in the application of the test-negative design that deserve further examination
Research
S. aureus colonization in healthy Australian adults receiving an investigational S. aureus 3-antigen vaccineBased on descriptive analyses of this small study, S. aureus 3-antigen vaccine vaccination did not impact S. aureus acquisition or carriage
Research
Effectiveness of Palivizumab against Respiratory Syncytial Virus: Cohort and Case Series AnalysisPalivizumab appeared effective for reducing virologically confirmed respiratory syncytial virus in this high-risk cohort
Research
B Part of It School Leaver protocol: an observational study to assess the impact of a meningococcal serogroup B vaccine programme on carriage of Neisseria meningitidisThis study will assess the impact of MenB vaccine (4CMenB) on carriage prevalence in school leavers in South Australia
Research
Safety and immunogenicity of pneumococcal conjugate vaccines in a high-risk population: a randomised controlled trial of PCV in Papua New Guinean infantsInfant vaccination with 3 doses of PCV10 or PCV13 is safe and immunogenic in a highly endemic setting
Research
Combination of clinical symptoms and blood biomarkers can improve discrimination between bacterial or viral community-acquired pneumonia in childrenCombining elevated CRP with the presence or absence of clinical signs/ symptoms differentiates definite bacterial from presumed viral pneumonia better than CRP alone
