Search
News & Events
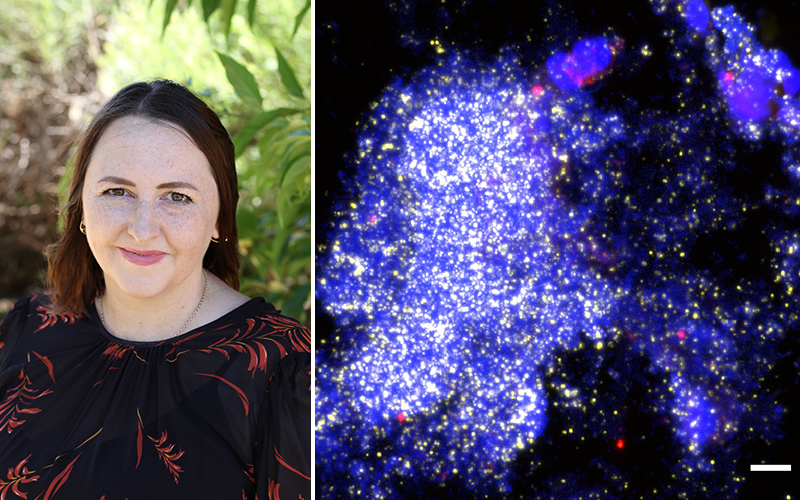
Bacterial slime causing persistent wet coughs for childrenResearchers using powerful microscopes have identified bacterial slime in the lungs of some children with persistent wet coughs.

News & Events
Major grant awarded to tackle antibiotic resistanceVital research aiming to improve the treatment of potentially deadly Group A Streptococcus (Strep A) has been awarded $820,000 in the latest round of National Health and Medicine Research Council’s Ideas Grants.

News & Events
Raine Foundation grants to support key child health researchThree outstanding young researchers from The Kids Research Institute Australia have been named Raine Fellows and received valuable Raine Priming Grants to support their child health research.
Research
STopping Acute Rheumatic Fever Infections to Strengthen Health (STARFISH)STopping Acute Rheumatic Fever Infections to Strengthen Health (STARFISH) brings together a diverse and multidisciplinary research team to investigate the most effective environmental health initiatives (EHIs) aimed at reducing Strep A infections and prevent Acute Rheumatic Fever (ARF).
Latest news & events at the Wesfarmers Centre of Vaccines & Infectious Diseases.

Research Theme
First Nations Health and EquityAboriginal health is everyone's business. The needs of Aboriginal and Torres Strait Islander families and kids is integrated into all relevant areas of our work. Improving the health and wellbeing of Aboriginal and Torres Strait Islander kids and families is an overarching priority for every team at The Kids.

News & Events
Skin infections send eight out of every 100 Aboriginal babies to hospitalIn a WA first, researchers from The Kids Research Institute Australia have shown that Aboriginal babies are 22.5 times more likely to be treated for skin infections than non-Aboriginal babies.
Research
What goes up must come down: dynamics of type 1 interferon signaling across the lifespanType 1 interferons (T1IFNs) are typically expressed in low concentrations under homeostatic conditions, but upon pathogenic insult or perturbation of the pathway, these critical immune signaling molecules can become either protectors from or drivers of pathology. While essential for initiating antiviral defense and modulating inflammation, dysregulation of T1IFN signaling can contribute to immunopathology, making it and its associated pathways prime targets for immune evasion and disruption by pathogens.
Research
Prevention of rheumatic heart disease in New Zealand: High-dose subcutaneous benzathine penicillin is cost-saving compared with traditional intramuscular injectionsAcute rheumatic fever is a preventable condition that can lead to chronic illness and early death. Standard prevention with 4-weekly intramuscular (IM) benzathine penicillin G (BPG) injections for ≥10 years may be associated with poor adherence. High-dose 10-weekly subcutaneous penicillin injections (SCIP) may improve adherence by reducing injection frequency.
Research
Collecting behavioural data across countries during pandemics: Development of the COVID-19 Risk Assessment ToolTools that can be used to collect behavioural data during pandemics are needed to inform policy and practice. The objective of this project was to develop the Your COVID-19 Risk tool in response to the global spread of COVID-19, aiming to promote health behaviour change. We developed an online resource based on key behavioural evidence-based risk factors related to contracting and spreading COVID-19. This tool allows for assessing risk and provides instant support to protect individuals from infection.
