Investigators: Prof Robert S Ware, Prof Harm A W M Tiddens, Barry S Clements, David S Armstrong, Prof Hiran Selvadurai, Dr Andrew Tai, Dr Peter J Cooper, Prof Catherine A Byrnes, Dr Yvonne Belessis, Prof Claire Wainwright, Prof Adam Jaffe, Prof Philip Robinson, Lisa Saiman, Prof Peter D Sly, and the COMBAT CF Study Group.
Partners: The COMBAT CF clinical trial was sponsored by The Kids Research Institute Australia and run in partnership with the University of Queensland across eight different CF clinics in Australia and New Zealand.
Project description:
For people with cystic fibrosis (CF) lung disease begins very early in life, even before respiratory symptoms develop in some children. Worsening lung disease leads to adverse clinical outcomes, such as exacerbations of respiratory symptoms, hospital admissions and reduced quality of life. Inflammation in the lung is present in children with CF from three months of age and occurs even without airway infections. High-dose ibuprofen is the only currently recommended anti-inflammatory medication for CF, and it is not recommended for use in infants or preschool aged children. With newborn screening now a common practice worldwide, there are opportunities for early treatment or intervention.
The COMBAT CF team, co-led by the previous Director of the Wal-yan Respiratory Research Centre at The Kids, and Professor Peter Sly from the University of Queensland, looked at whether a commonly prescribed antibiotic called azithromycin could prevent or delay the onset of lung disease in infants newly diagnosed with CF by newborn screening. Azithromycin also has anti-inflammatory properties, so we anticipated treatment with this medicine would reduce levels of inflammation in the lungs.
The COMBAT CF clinical trial began in 2012 and ended in 2021, having recruited 130 babies with CF across eight CF clinics in Australia and New Zealand. Participants had a 50:50 chance of receiving either azithromycin or a placebo three days a week from their diagnosis until they turned three years old. We looked at the levels of inflammation in their lungs from samples taken during a clinical procedure called bronchoalveolar lavage and assessed any structural changes in their lungs by looking at images from chest CT scans. We also documented how often they were hospitalised for respiratory illness, how many courses of antibiotics they took, and their quality of life as reported by their parents.
We found that azithromycin did not affect structural lung changes associated with CF lung disease, however it did have significant positive effects on other health outcomes!
We found that treatment with azithromycin leads to:
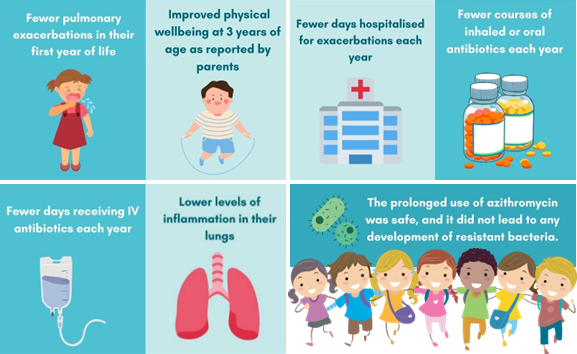
Our findings from the COMBAT CF study were published in high-impact medical journal The Lancet Respiratory Medicine in 2022. While azithromycin had no effect on structural lung disease, another clinical trial led by Professor Stephen Stick treated preschool children who have CF with hypertonic saline twice a day for 48 weeks. This study, called SHIP-CT, found hypertonic saline to be the first treatment to positively affect the progression of structural lung disease in children with CF. You can find out more about the SHIP-CT study here.
This important work would not have been possible without the 130 families who took part in the study. We thank them for their time and their significant contribution to CF research.
External collaborators:
- Prof Peter D Sly, Child Health Research Centre, The University of Queensland, Brisbane,
- Prof Robert S Ware, Griffith University, Gold Coast,
- Prof Harm A W M Tiddens, Erasmus University Medical Centre, Rotterdam,
- Dr David S Armstrong, Monash Children's Hospital, Melbourne,
- Prof Hiran Selvadurai, The Children's Hospital at Westmead, Sydney,
- Dr Andrew Tai, Women's and Children's Hospital, Adelaide,
- Dr Peter Cooper, The Children's Hospital at Westmead, Sydney,
- Prof Catherine A Byrnes, Starship Children's Health, Auckland,
- Dr Yvonne Belessis, Sydney Children's Hospital, Sydney,
- Prof Claire Wainwright, Queensland Children's Hospital, Brisbane,
- Prof Adam Jaffe, Sydney Children's Hospital, Sydney,
- Prof Philip Robinson, The Royal Children's Hospital, Melbourne
- Dr Lisa Saiman, New York Presbyterian Hospital, New York
Funders: The Cystic Fibrosis Foundation
